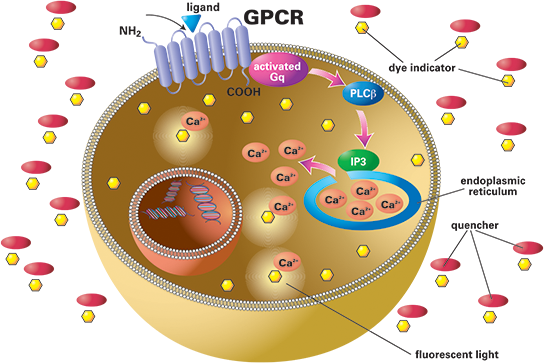
The FLIPR calcium assay allows for simultaneous detection of GPCRs and ion channels in one assay. Cells are loaded with calcium-sensitive dye, and when activated by an agonist, there is an increase in fluorescence signal.
Rhod-4 AM is the brightest red calcium indicator available for HTS screening. Once inside cells, lipophilic blocking groups break apart, allowing Rhod-4 AM dye to bind calcium more readily and increase fluorescence.

Detection of GPCRs
GPCRs and ion channels coupled to Ca2+ can be important drug targets; modulation of their signalling pathways is one approach to treating disease. Fluorescent imaging plate reader (FLIPR) technology to measure calcium ion flux has proven invaluable for high-throughput screening (HTS) and lead optimisation applications targeting such targets.
GPCRs that naturally couple to Gq produce a ligand-induced increase in intracellular calcium that can be measured using a calcium-sensitive dye. GPCRs that do not signal through Gaq can still be “turned on” to produce calcium readouts using promiscuous or chimeric G protein constructs that overexpress this protein in cells.
The FLIPR calcium assay kit utilises advanced fluorophore and masking technology to increase the dynamic range, signal-to-background ratio, and throughput of an assay, enabling better detection of agonists, antagonists, and allosteric modulators in one assay. Two or three additions may be used, depending on what needs to be detected. Furthermore, with its in-tip dilution capability, the FLIPR Tetra System eliminates intermediate dilution plates off-line and enhances throughput significantly.
Detection of Ion Channels
Patch clamp technology remains an industry standard for characterising ion channels; however, its time-intensive nature precludes its use in high-throughput screening (HTS). The FLIPR system has proven itself capable of supporting effective HTS screening for ion channel targets by quickly loading cell clones with Fluo-3AM fluorescent dye, stimulating Ca2+ flux using known agonists, and measuring fluorescence responses from individual cell clones within minutes.
Fluo-3 or Fluo-4 no-wash cell-permeable calcium-sensitive dye is ideal when using the FLIPR system to detect ion channel blockers; its rapid sub-second Ca2+ inflow rates have no bearing on the redistribution of dye in FLIPR, where signal generation typically takes minutes before becoming detectable by the FLIPR system.
Probenecid should always be included in dye solutions used when working with CHO cells (5 mM is sufficient). This will prevent dye release into the buffer during washing steps. Sodium azide may also help increase the assay window and signal durability.
Detection of Allosteric Modulators
GPCRs and ion channels regulated by changes in intracellular calcium have become drug discovery targets. A fluorescent imaging plate reader (FLIPR) allows high-throughput screening of these compounds with ease.
The FLIPR is an instrument with a cooled CCD camera designed to simultaneously measure fluorescence from each well of a microplate at sub-second intervals and monitor real-time calcium responses.
Conventional FLIPR calcium assays rely on membrane-permeable dyes that bind Ca2+ in cells, leading to an increase in their fluorescence. Unfortunately, this method has two significant drawbacks: (1) large downward spikes during liquid transfer can obscure an agonist response, and (2) cleaning dye-loaded cells requires extra time and reagents.
To overcome these limitations, a no-wash FLIPR Calcium Assay Kit was created that eliminates the need for washing while providing more accurate measurements of Ca2+ responses. It uses the patented quenching dye (1R,2R)-2-PCCA that works to decrease background noise in cell-based mobilisation assays of Ca2+ mobilisation.
Detection of Agonists
As part of GPCR FLIPR assays, agonist detection is typically achieved through one addition. This approach reduces the number of plates, cells, reagents, and robotic runtime needed for screening; however, it may lead to false positives when screening inhibitors as well as an absence of detection for allosteric modulators if added without glutamate (Figure 9A).
An FLIPR assay protocol has been designed that allows simultaneous screening for both agonist and antagonist agents on a single plate, drastically cutting back on the number of plates, cells, and reagents required as well as significantly cutting screening time in half.
Protocol Description: To conduct an FLIPR assay using different test compounds, first conduct a concentration response experiment to identify which cell line and dye concentration results in an adequate signal window for FLIPR assay plates; subsequently, conduct another FLIPR assay using those same plates but introducing new test compounds: response in either read indicates either non-desensitising agonist activity or allosteric modulatory properties, respectively.
Detection of Antagonists
GPCRs and ion channels that release Ca2+ represent an essential class of drug targets, and in HTS, these targets can be identified using intracellular calcium-sensitive dyes and the FLIPR instrument. Assays performed against such targets mimic those for G-protein-coupled receptors; similar curve classes can be obtained (Table 1).
As variations in cell density can have an impactful impact on basal Ca2+ mobilisation, dye-loading experiments must be performed to establish which combination of cell line, agonist, and concentration of dye creates an unambiguous signal window in FLIPR. Cultured cells or frozen aliquots may be brought back out on test day for plating.
Preparing the target ligand dilution series requires adding it directly to each well during the 60-minute waiting period, using an assay buffer with no ethanol or methanol, as this may interfere with the FLIPR readout. Before inserting plates into FlexStation for testing, shake them briefly prior to shaking to eliminate air bubbles and ensure proper mixing of solution.
